Beryllium is a steel grey hard and brittle metal with relatively low density, high thermal capacity and high sound conductivity. Due to its interesting physical-chemical characteristics, it has many uses in special materials used for example in aerospace applications or electronics. Main drawback of beryllium and its compounds is really high toxicity.
For the beryllium, 12 isotopes is known. 9Be is the only stable isotope, the rest undergoes radioactive decay with half-life ranging from 5x10-21 sec in case of 6Be to 1.39x106 years in case of 10Be. The radionuclide which is in our focus today is 7Be with half-life 52.2 days, which decays by electron capture to 7Li. Created 7Li nucleus is in excited state and releases gamma quantum at 477 keV in order to get into the ground state.
Main source of this radionuclide in our environment is spallation reaction caused by energetic cosmic radiation producing atmospheric cascade with high energy nucleonic components colliding with atoms of nitrogen or oxygen in upper atmosphere [1]. Unlike the fission, spallation is more like shattering of original nucleus into many smaller parts. To split atom nucleus apart by spallation, some good projectile with high load of energy is really needed and protons with energies in order of tens to hundreds gigaelectronvolts (GeV) are good for this purpose. The mechanism of the collision is depicted on the image below.
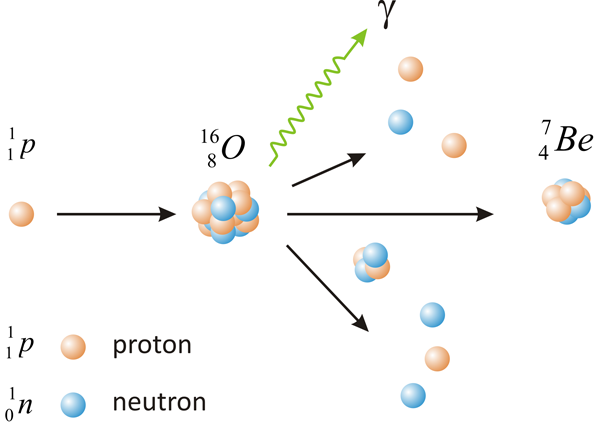
Even if it does not seem to happen, we all meet 7Be nuclide in everyday life, since 7Be is brought to the ground with each rain, where this Be isotope absorbs on the soil particles in thin top layer. But one do not have to worry about radioactive fallout and cumulative contamination, due to relatively short half-life of 7Be (55.2 d) and its extreme low concentration and activity in rain water (~ units of Bq/L). Beryllium-7 even found its value as a tool for studiing of atmospheric transport of air masses or in the sedimentology [2, 3].


Method which I used for pre-concentration of 7Be from rain water before a measurement uses co-precipitation of 7Be with MnO2 from the solution. Procedure is described in the work of Short et. al [4]. Briefly: 4.45 L of rain water collected in a plastic canister during overnight storm at 22.6.2017 was transferred to a clean glass beaker previously cleaned with surfactant and treated with 50% (w/w) nitric acid. To the volume of water, 4.45 mL of potassium permanganate (KMnO4, 0.3 M) was added. pH was adjusted with ammonia to the value 10.2 and solution was mixed for 5 minutes at 400 RPM. In the next step 4.45 mL of manganese(II) sulfate (MnSO4, 0.3 M) was added and mixture was mixed at 400 RPM for another 20 minutes, in order to carry reaction out and obtain MnO2 precipitate. After that suspension was sedimented, supernatant decanted and MnO2 filtered through 0.4 um filter. MnO2 precipitate was dried at 105°C, weighted (310 mg) and adjusted in thin layer with glue in plastic Petri dish (diameter 55 mm).
| Sample | 7Be from rainwater on MnO2 carrier |
| Gamma spectrometer | Scintillix SCGS-01 |
| Scintillation probe | NaI(Tl) 3" |
| Integration time | 128094 sec (35.6 h) |
| Background correction | No/Yes |
| Shield | Lead |
| Software | Theremino v.7.2 |
Radiation from the sample was integrated for more than 35 hours under a lead shield. The result of measurement can be seen below.
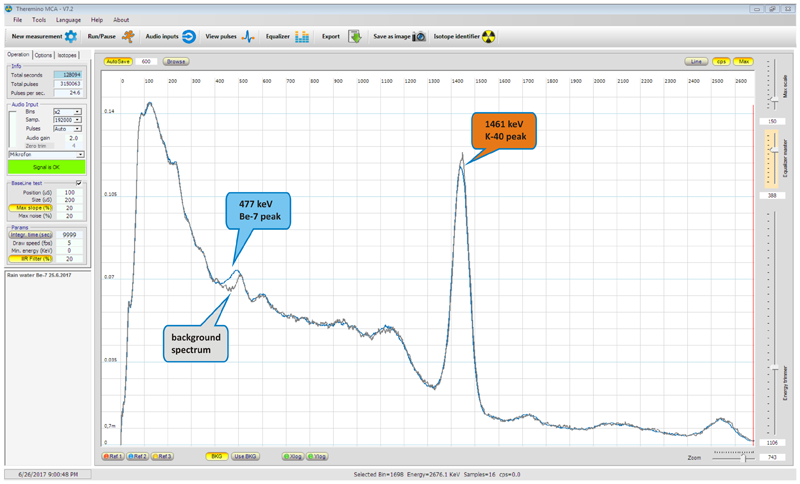
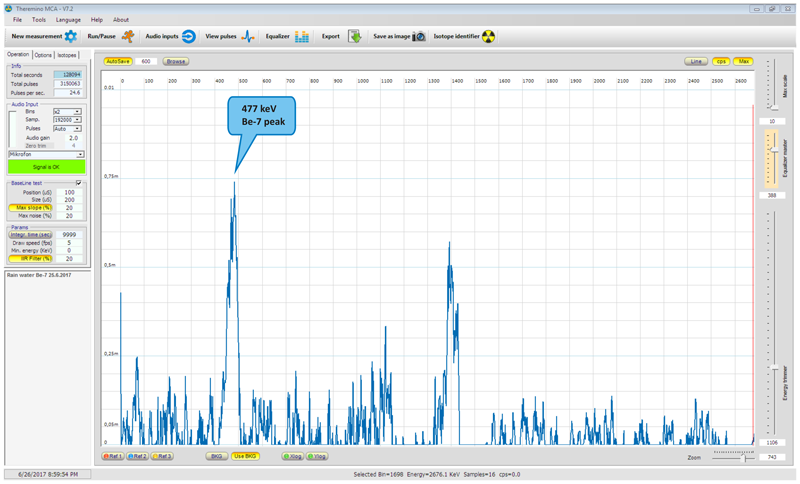
Upper spectrum shows overlay of background spectrum and 7-Be sample spectrum. Lower image shows result corrected for background (background subtracted). At the first sight it can seem that the subtracted spectrum is of low quality, but what is important to realize is the fact, that very low activity was measured (several Bq/L) and actual weight of 7Be calculated in the collected rain water was in order of 1x10-16 g (hundreds of attogram), so in this light I consider the result as excellent demonstration of device sensitivity.
References:
[1] Yoshimori M. Production and behavior of beryllium 7 radionuclide in the upper atmosphere. Advances in Space Research. 36(5), 2005, pp922-926
[2] Raisbeck, G. M., F. Yiou, M. Fruneau, J. M. Loiseaux, M. Lieuvin, and J. C. Ravel. Cosmogenic Be-10/Be-7 as a probe of atmospheric transport processes. Geophysical Research Letters, 8, 1981, pp1015–1018.
[3] Blake W. H., Walling D. E., He Q. Using cosmogenic Beryllium-7 as a tracer in sediment budget investigations. Geografiska Annaler: Series A, Physical Geography, 84, 2002, pp 89-102.
[4] Short D.B., Appleby P.G., Hilton J. Measurement of atmospheric fluxes of radionuclides at a UK site using both direct (rain) and indirect (soils) methods. International Journal of Environment and Pollution, 29(4), 2007, pp392-404

