Lutetium is silvery grey metal element belonging to the group of rare earth metals (lanthanides) as its last member which closes it. Unlike some other lanthanides, lutetium is relatively stable at dry air, but is readily oxidized in humid environment and at higher temperatures. Lutetium has highest hardness (Brinnel - 893 MPa), density (9.84 g/cm3) and melting point (1663 °C) of the lanthanides. In the nature, it occurs as a mixture of two isotopes 175Lu and 176Lu with natural abundances 97.41 % and 2.59 % respectively. While the former is stable 176Lu is β- emitter with half life 3.78x107 years.
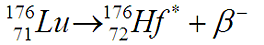
Emerging 176Hf* nucleus is in higher energy state and emits series of gamma quanta with energies 88.3, 201.8, 306.8 and 401.0 keV in order to get to the ground state. A total of 34 radioisotopes of Lutetium is known, but except 176Lu the rest is artificial and has orders of magnitude shorter half-life than naturally occurring radioisotope 176Lu, which half-life is nearly 3x longer than age of the universe, according to our presentday knowledge.
Lutetium and its compounds has found its use for example in petrochemical industry as a catalysts, it is a component of LYSO scintillation crystals (Lutetium-yttrium oxyorthosilicate), is used in electrotechnics in LED diodes. Radioactive isotopes of lutetium has found its utility for example in medicine, like 177Lu in radiotherapy. Naturally occurring radioisotope 176Lu is used for dating of rocks and meteorites.
The isotope which is of our interest today is naturally occurring 176Lu as a part of lutetium metal. Nice sample of this metal weighting 0.89 g with purity at least 99.999%, which was used for measurement, is sealed in glass ampoule under argon to prevent its oxidation. Despite the really long half-life, lutetium metal is little "hot" if compared with other similar stuff. When I prepared the measurement with Scintillix, I expected that I will see usable spectrum after several hours of data acquisition, but what was my surprise, when after couple of seconds, two characteristic peaks at 201.8 and 306.8 keV popped up above the noise level.
| Sample | Lutetium metal (0.89 g, 99.999% purity, 2.59% 176Lu) |
| Gamma spectrometer | Scintillix SCGS-01 |
| Scintillation probe | NaI(Tl) 3" |
| Integration time | 79200 sec (22h) |
| Background correction | Yes |
| Shield | Lead |
| Software | Theremino v.7.2 |
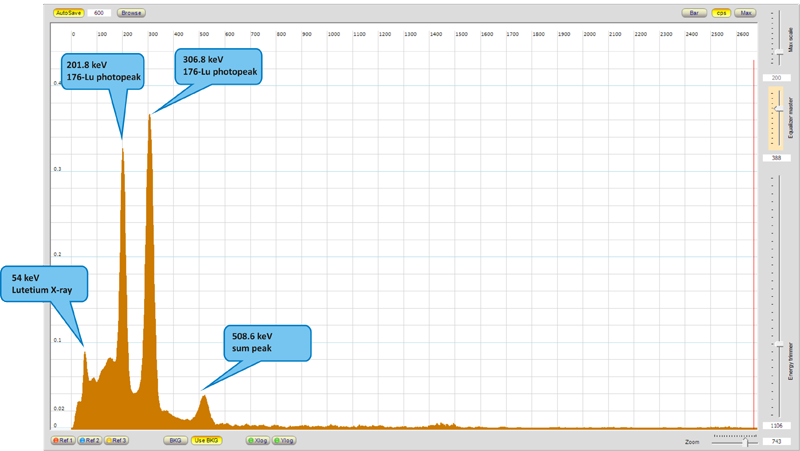
As I mentioned above, natural abundance of 176Lu is 2.59 mol.%. Sample of lutetium metal which I took for the measurement weighting 0.98 g should contain about 23 mg of radioactive isotope 176Lu. As we can see from the spectrum, this amount is more than sufficient to unequivocally confirm presence of this isotope in the sample.

